Better process, better results.1
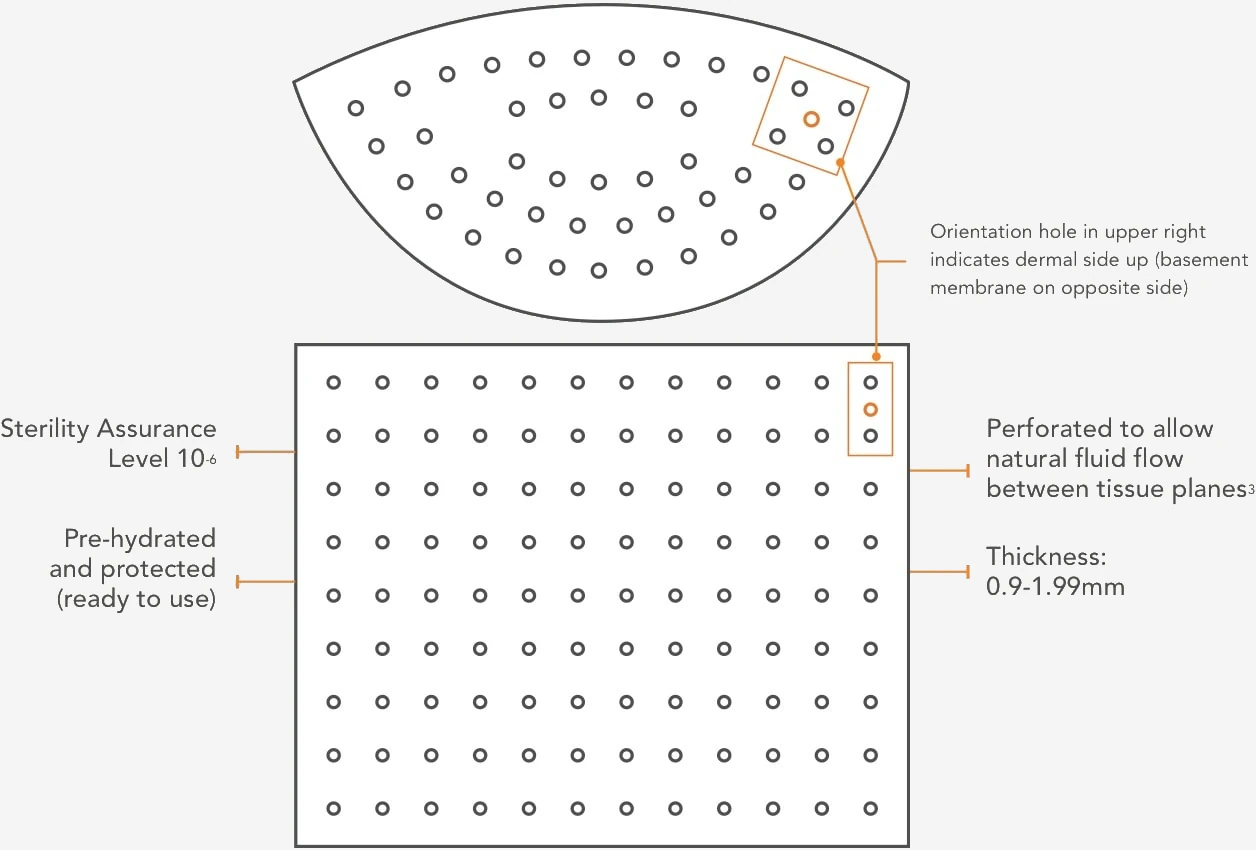
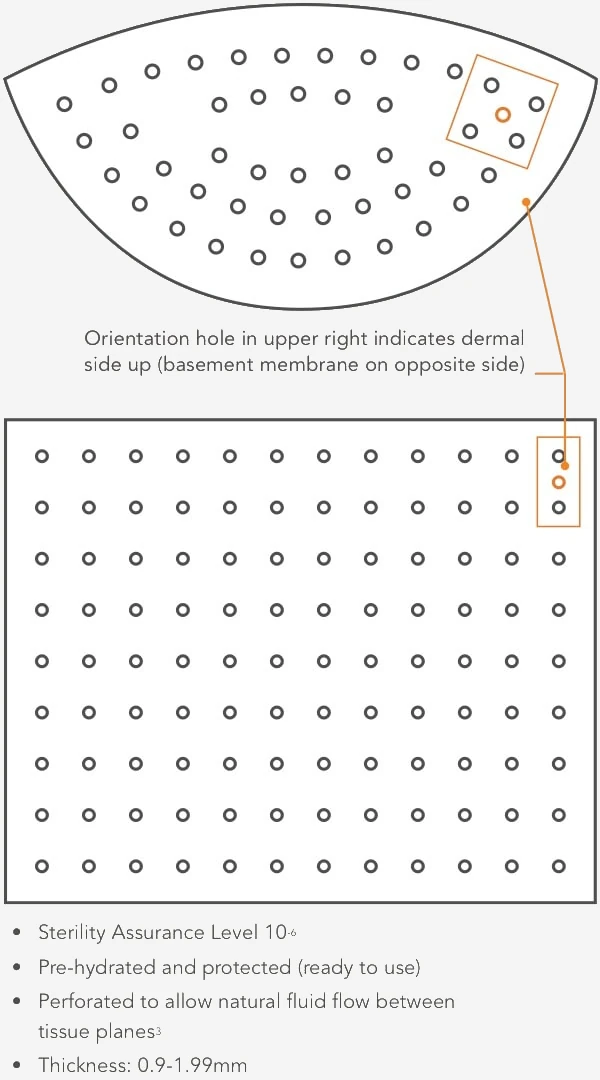
SimpliDerm’s proprietary, patented and gentle process achieves a sterility assurance level (SAL) of 10-6 with minimal impact to the extracellular matrix and key growth factors. This innovative process results in better handling and performance, healthy inflammatory response and rapid tissue integration.1, 2
Designed for consistency
Designed to meet the needs of board-certified plastic surgeons, the SimpliDerm process prioritizes consistency in thickness within and between pieces for optimal results.
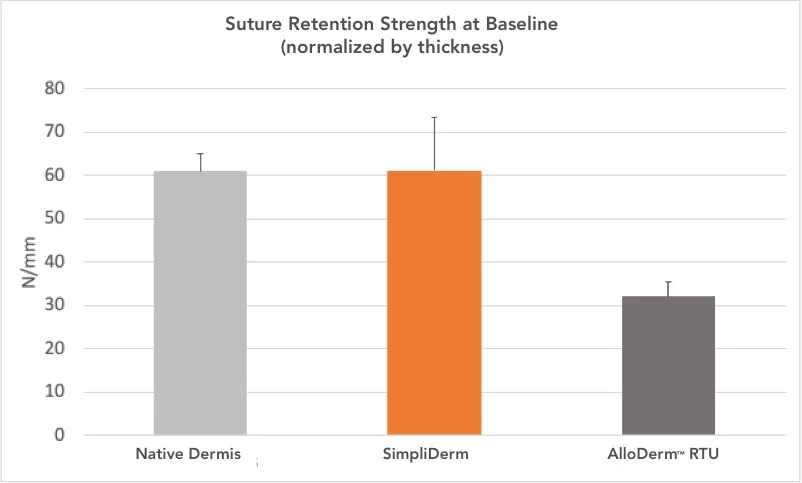
Greater tissue strength2
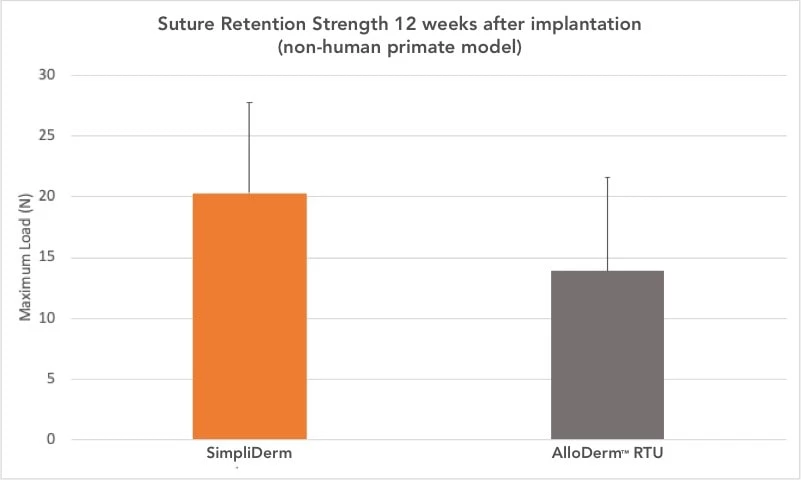
Better Handling and Performance2
SimpliDerm’s structural composition is similar to native dermis and exhibits greater strength than other hADMs, while maintaining pliability.2
Learn more about SimpliDerm
- Ji H, et al. Characterization of Inflammatory and Fibrotic Aspects of Tissue Remodeling of Acellular Dermal Matrix in a Nonhuman Primate Model. Plast Reconstr Surg Glob Open. 2021 Feb 16;9(2):e3420.
- LT-0032 Rev. 01, “SimpliDerm Scientific Overview”
- IFU-0027 Rev. 04, “SimpliDerm IFU”
Aziyo® and SimpliDerm® are registered trademarks of Elutia Inc. All other registered trademarks are property of their respective owners.
SimpliDerm is to be used for the repair or replacement of damaged or insufficient integumental tissue. It may also be used for the repair, reinforcement or supplemental support of soft tissue defects or any other homologous use of human integument. Each package of SimpliDerm is intended for use in one patient on a single occasion by a licensed physician.
CONTRAINDICATIONS
Use of SimpliDerm in patients exhibiting autoimmune connective tissue disease is not recommended. SimpliDerm should not be used in patients with sensitivities to processing agents (see WARNINGS).
WARNINGS
Potential adverse effects that may result from placement of SimpliDerm include, but are not limited to wound or systemic infection; seroma; dehiscence; hypersensitivity; allergic or other immune response; sloughing or failure of the graft; and disease transmission.
Trace amounts of processing agents include, but are not limited to: gentamicin, maltodextrin and trehalose.
Do not re-sterilize or reuse.
The FDA has not cleared or approved any ADM product for use in breast reconstruction.
Extensive medical screening procedures have been used in the selection of all tissue donors (see Donor Screening and Testing). Due to limitations in testing technology, testing and donor screening cannot totally eliminate the risk that human source material may transmit infectious agents or diseases.
