A new standard in cellulite treatment
Fast, real results that last1

Avéli named "Best Minimally Invasive Cellulite Treatment" by NewBeauty

Avéli named "Best Minimally Invasive Cellulite Treatment" by NewBeauty
Cellulite is problematic
- Up to 90% of women have cellulite2
- A significant majority are bothered by it3
- Women are frustrated and disappointed because nothing has worked3
- Over 70% willing to pay a minimum of $3K and ~50% willing to pay more than $4K up to $10K to treat it

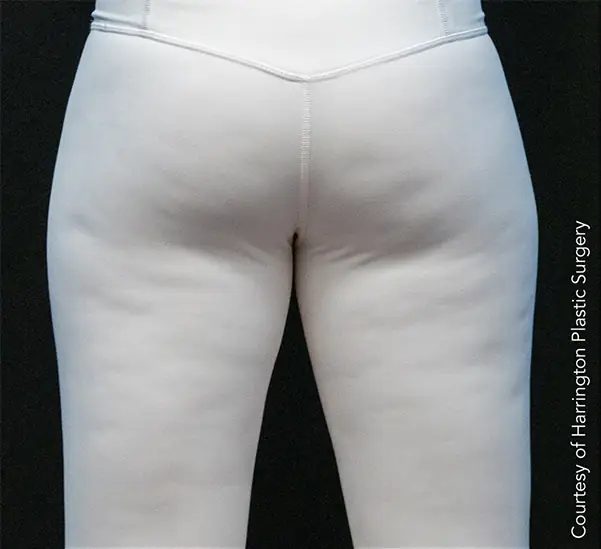
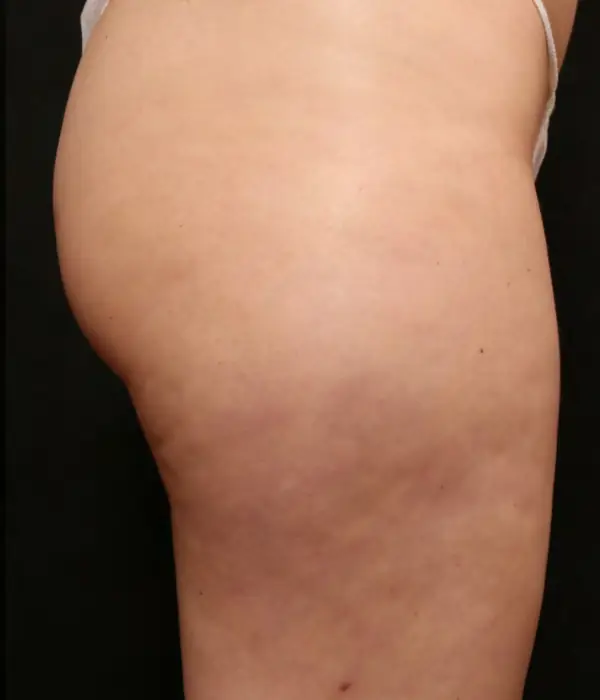
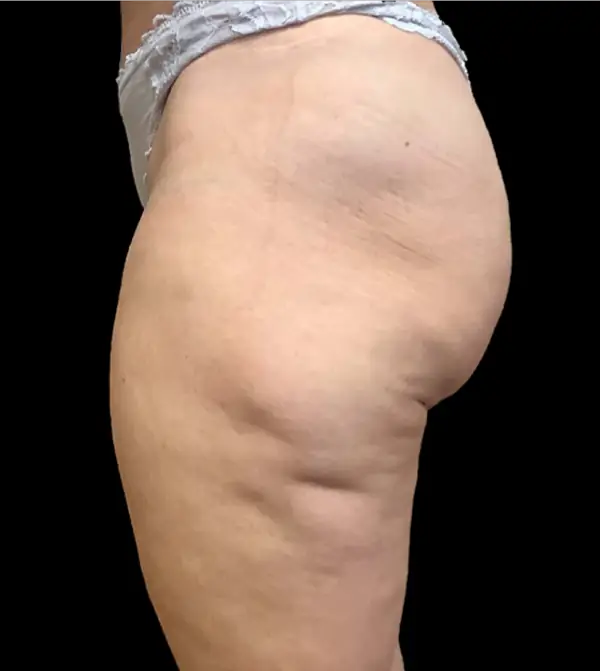
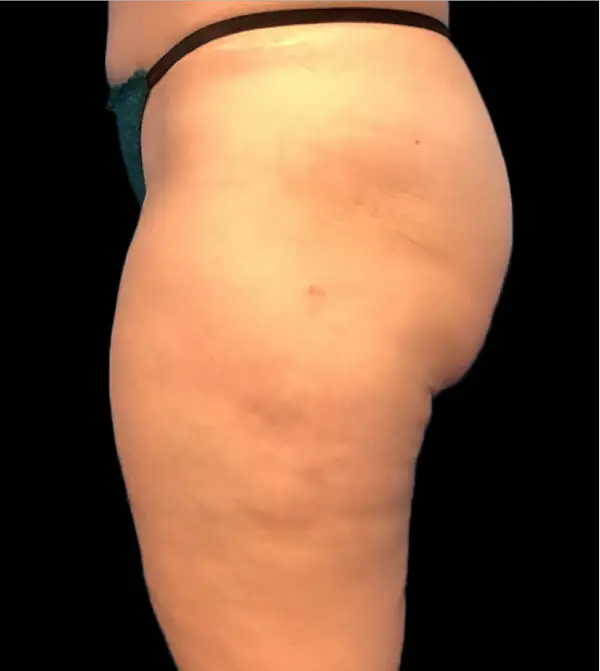
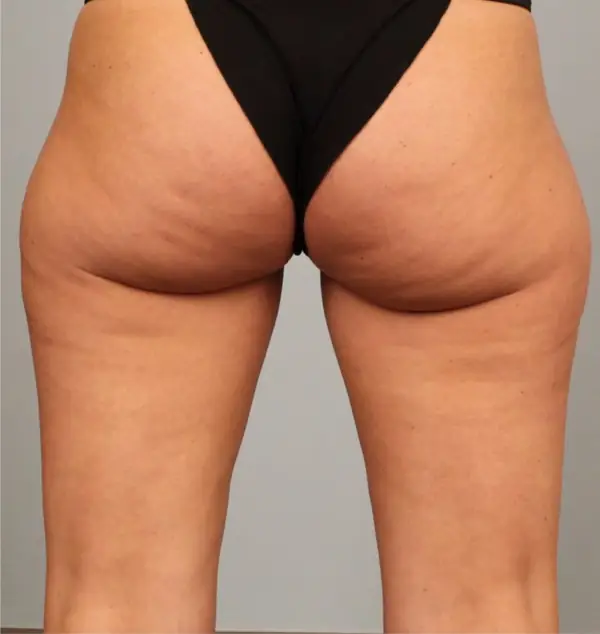
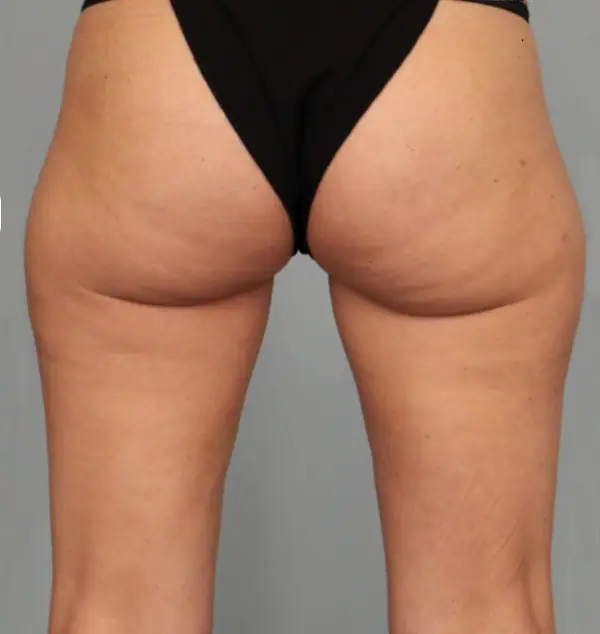
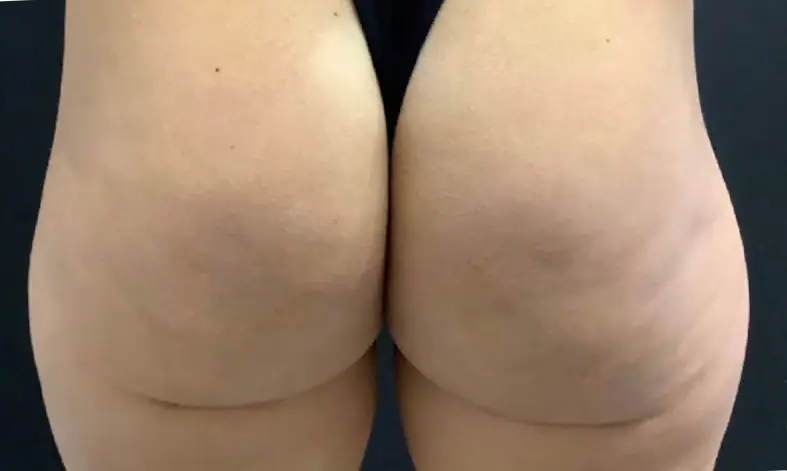
Avéli provides long-term reduction in the appearance of cellulite in both the buttocks and thighs in a single procedure.5
It’s the only cellulite device that identifies and releases the culprit septa with real-time confirmation.1,5
Precise, predictable and repeatable results
Identify
Complex webs of septa tensioning the skin
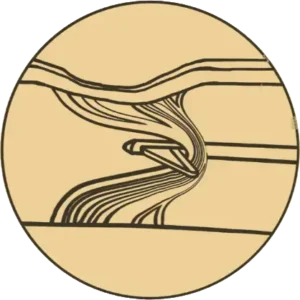
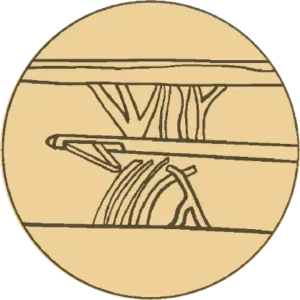
Release
Controlled and precise release
of fibrous septa causing cellulite
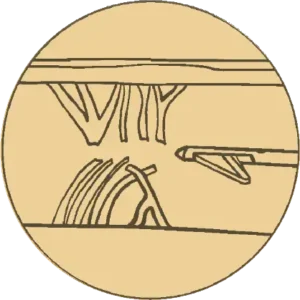
Verify
Confirmation of release of culprit septa structures
Avéli is profitable.
- Avéli offers dual practice opportunity as stand-alone
treatment or add-on to overall treatment plan.* - No captial required.
- Instantly accretive to the practice.
*Avéli has not been studied in combination with other treatments

Meaningful, visible results that last
Experience the Avéli® difference.
Find out how this one-of-a-kind procedure can enhance your practice.
Avéli® is indicated for long-term reduction in the appearance of cellulite in the buttocks and thigh areas of adult females as supported by clinical data demonstrating treatment benefits through one year of observation. Avéli is also indicated for soft tissue dissection during general and plastic surgical procedures. The most common side effects reported were mild pain within the first 24 hours and bruising and tenderness to the touch which typically resolved within 30 days. Some patients returned to normal activities within one day of the procedure and most within a week. As with any medical procedure, there are risks associated with the procedure. Refer to the Instructions for Use at myaveli.com/IFU for full safety information.
CONTRAINDICATIONSAvéli should not be used on patients who have (or who are):
- Coagulant disorders/on anticoagulant medications
- Diabetic
- Had recent surgery (6 weeks)
- Pregnant
- Severe anemia
- Skin infections/open lesions
- Tumors
- Uncontrolled hypertension
- Varicose veins (in the area of treatment)
- Stevens WG, et al. Multicenter Pivotal Study Demonstrates Safety and Efficacy of a New Cellulite Procedure: Final Results at 12 Months [published online ahead of print, 2022 Nov 10]. Aesthet Surg J. 2022; sjac291
- Emanuele E. Cellulite: advances in treatment: facts and controversies. Clin Dermatol. 2013; 31(6):725-30.
- Data on file. Revelle Aesthetics, Inc. 2021. Patient Quantitative Research Report, June 2021.
- Revelle patient qualitative research, June 2021. N=500 women aged 25-60, concerned by their cellulite 38 What would you expect Procedure C to cost? Use the slider below to reflect your cost expectation.
- Avéli Precision Cellulite Device. Instructions for Use. Mountain View, CA: Revelle Aesthetics, Inc; 2024.