The only system with a surfactant shown to enhance the survival of fat cells1
The only FDA-cleared system with Auraclens™ lipoaspirate wash
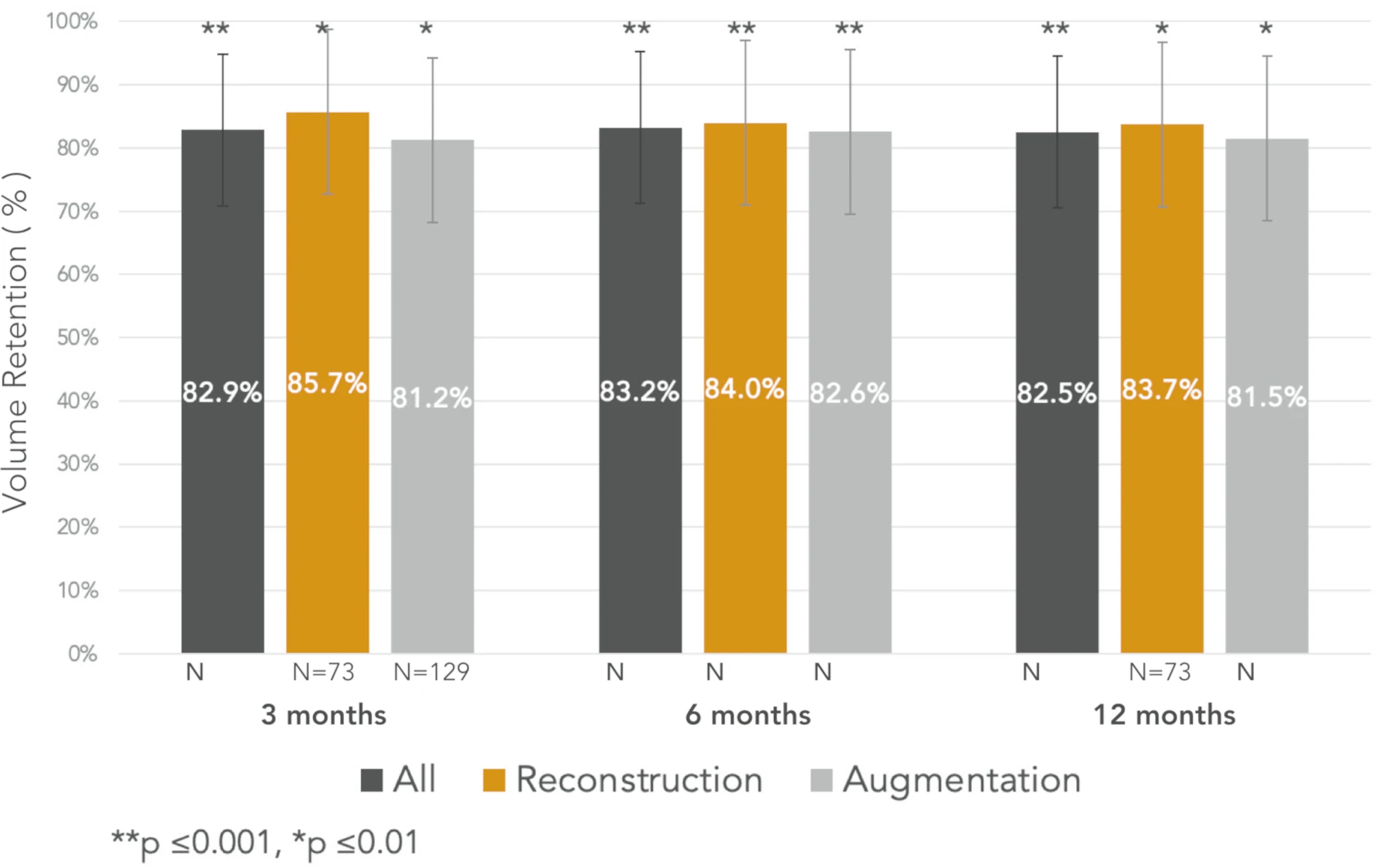
Greater than 80% average fat retention at 3, 6, and 12 months2
Validated by an ongoing multi-center prospective clinical study3
Easy. Versatile. Effective.
Easy-to-use
in-line system
Single-use device requires less than 10 minutes processing time.4
High capacity
The ability to process large amounts of lipoaspirate enables use in a broad range of cosmetic procedures.

200 Micron mesh filter
Allows unwanted fluids to pass through while retaining viable fat cells.
Super absorbent foam
Dense pore design allows for efficient absorption of unwanted fluids, leaving highly concentrated fat behind.
Viality by Tiger Aesthetics can further enhance the viability and survival of the transferred fat cells, resulting in better outcomes, with predictable results.2
Greater than 80% average fat retention at 3, 6, and 12 months2
Consistent Volume Retention2
Study Design: Multicenter 12 Month Volume Retention3
SITES
14
PATIENTS
Up to 200
PROCEDURES
Reconstruction
2nd + 3rd stage reconstruction
Augmentation
primary & revision/mastopexy (fat only, implant + fat, explant + fat)
ENDPOINTS
Volume retention and patient satisfaction at 12 months with follow-ups at 1, 3, 6 months
CORE LAB ASSESSMENT
Performed by Canfield with Vectra 3D imaging; no point estimates by surgeons
AuraClens: a surfactant shown to enhance the viability of fat cells
Cell Viability with Normal saline vs P188 treatment at six weeks1
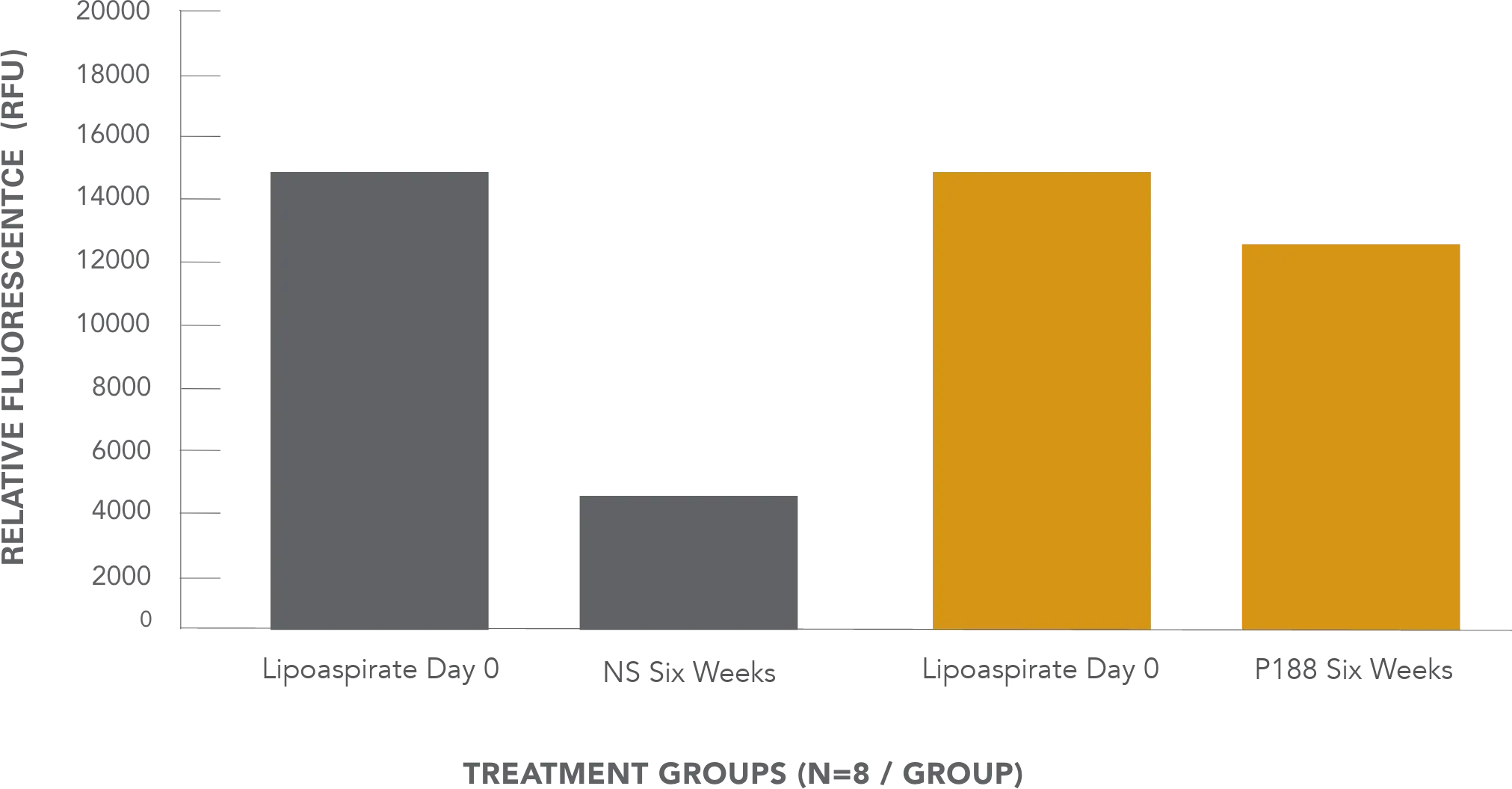
Fig 1. Differences in live cell signal between P188-treated grafts and normal saline-treated grafts were statistically significant. P188-treated grafts demonstrated three times the live cell signal of normal saline-treated samples, which demonstrated only 33 percent of its day-0 live signal compared with 87 percent in P188-treated samples.
"...poloxamers with membrane-sealing capability, can increase graft survival. Among these poloxamers, [AuraClens] demonstrated statistically significant improvement in apoptosis, graft survival by weight, cell viability, DNA content, and histology."1
Experience a new standard in fat transfer.
Wash away the barriers to fat retention.
- Medina MA 3rd, et al. Polymer therapy: a novel treatment to improve fat graft viability. Plast Reconstr Surg. 2011 Jun; 127(6):2270-2282. doi: 10.1097/PRS.0b013e3182139fc1. PMID: 21617461. Images and selections reprinted with permission.
- Shridharani, S, et al. Preliminary Results from the Prospective Multicenter Volume Retention Study of Viality™ with AuraClens™ Lipoaspirate Wash System Support the Effectiveness of Enhanced Viability Fat Transfer. 2024; 1-8. [Sientra White paper R2].
- Tiger Aesthetics, Inc. AuraClens™ System in Processing of Lipoaspirate for Autologous Fat Grafting to the Breast (AuraGen). ClinicalTrials.gov. NCT05258305. Updated May 15, 2023. Accessed October 11, 2023. https://clinicaltrials.gov/study/NCT05258305.
- An Y, et al. Comparative Analysis of Two Automated Fat-processing Systems. Plast Reconstr Surg Glob Open. 2020; 8(1):e2587. Published 2020 Jan 17. doi:10.1097/GOX.0000000000002587
